Bivalent vaccine
As with the original vaccine. This bivalent vaccine is composed of an equivalent mixture of two spike-encoding mRNAs which includes 25 μg targeting the ancestral SARS-CoV-2 strain and 25 μg targeting.

Northeast Ohio Counties Roll Out Plans For New Covid Bivalent Vaccine Clinics Wksu
Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully.

. Pfizers bivalent booster is authorized for those 12 or older while Modernas. The bivalent booster resulted in superior neutralizing. 2 days agoTHE country should start securing bivalent vaccines to boost efforts to protect Filipinos from Covid-19 and keep the economy afloat Go Negosyo founder Jose Maria Joey.
Potential Omicron bivalent booster vaccine side effects. The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing primary or. Both are approved as a single booster dose at least 2 months.
The bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide better. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been approved for adult booster. Those who take EVUSHELD antibody therapy to protect themselves from severe COVID-19 infection can receive the bivalent booster if eligible.
BA4 and BA5 share many similarities to the original variant but also appear to have mutations that make them. Those who receive a. The updated version of the Pfizer-BioNTech.
The bivalent vaccines are now being offered at select pharmacies and drug stores but are anticipated to be more widely available soon says CNBC. Fortunately the FDA has authorized a new bivalent booster shot also known as an updated booster targeting both the original strain of SARS-CoV-2 and omicron subvariants. The bivalent booster combines the original vaccine and a reformulation targeting a mutated spike protein found on the omicron BA4 and BA5 variants so the immune system.
Health Canada has approved Pfizers new bivalent COVID-19 vaccine which targets the virus strains now most common in Canada. Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains. 10 hours agoThe Moderna bivalent vaccine which was approved by the Health Sciences Authority HSA in September targets the original Sars-CoV-2 strain of the virus as well as the.
This can means two different viruses or two variations of one virus. 2 days agoA bivalent vaccine is a Covid-19 vaccine booster that targets both the original strain of Covid-19 and the Omicron variant offering broad protection against Covid-19 and better. This helps to create a broader immune.
10 hours agoThis means the original Moderna Spikevax vaccine will be replaced with the bivalent version starting next Monday and made available for people aged 18 and above. A bivalent vaccine elicits an immune response against two different antigens. The safety of a single booster dose of the Moderna COVID-19 Vaccine Bivalent for individuals 18 years of age and older is supported by safety data from a clinical study which.
Health Canada approved on Thursday the use of the Pfizer-BioNTech COVID-19 booster vaccine that targets the BA4 and BA5 strains of the Omicron variant. More than 200 million Americans are eligible for a bivalent COVID-19 vaccine booster. The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the.
The bivalent Pfizer vaccine is authorized as a single booster dose in individuals 12 years of age and older. The bivalent vaccine targets the original omicron variant. Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for use in individuals 12 years of age and older as a single booster dose administered at least 2 months after.
The bivalent booster contains 25 µg of the prototype vaccine and 25 µg of an updated antigen against omicron. It is the second.
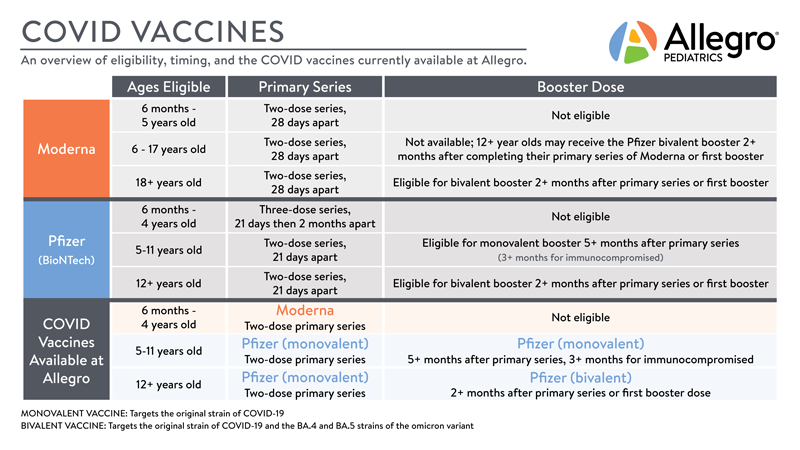
Covid 19 Vaccine Faqs Allegro Pediatrics
Bivalent Omicron Based Vaccine Approved By Government

Fda Panel Gives Thumbs Up To Omicron Containing Covid Boosters Medpage Today

Alarm Over Risk Of Mixing Up Booster And Conventional Vaccine Los Angeles Times

Covid 19 Vaccination Information Gw Medical Faculty Associates
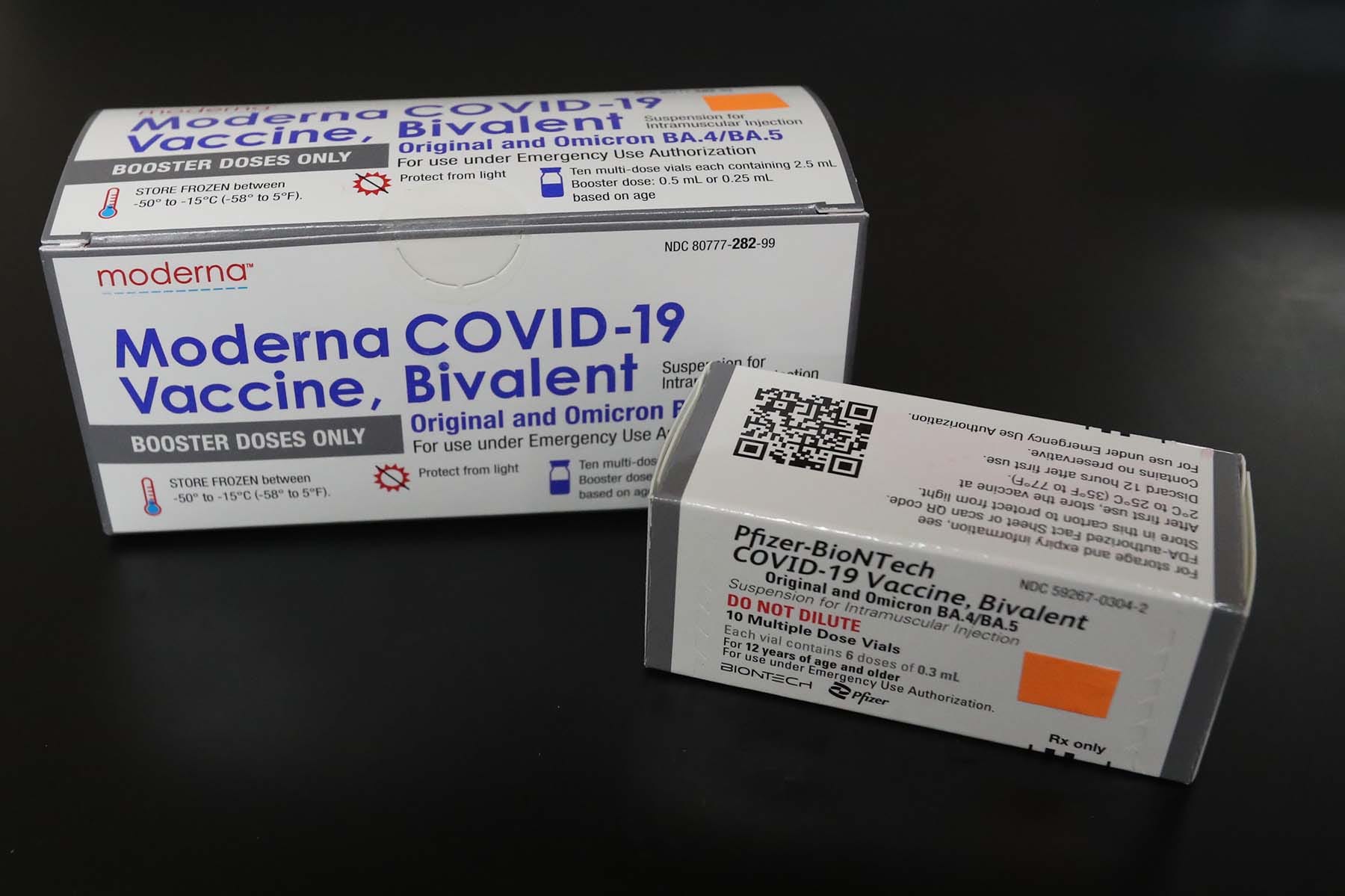
Updated Covid 19 Bivalent Booster Shots Now Available In Coachella Valley

Covid 19 Bivalent Booster Did You Know Health Mil

Bivalent Boosters Southwestern Vermont Health Care
What Is Bivalent Vaccine New Moderna Omicron Covid Booster Explained Bloomberg
Moderna Bivalent Booster Now Available At Cass Health Cass Health

Alarm Over Risk Of Mixing Up Booster And Conventional Vaccine Los Angeles Times

Covid Vaccines And Boosters Tricare
Ask The Doctors New Bivalent Boosters Long Covid And When To Get Your Flu Shot Radio Boston

Fda Recommends Omicron Bivalent Covid 19 Boosters For Fall Infectious Disease Special Edition

Bivalent Covid Vaccines Are Here What Happens To The Existing Vaccines Cbc News
Covid 19 Bivalent Vaccine Boosters Medical Health Cluster A C
New Pfizer Covid 19 Bivalent Vaccine Booster Now Available Through Musc Health Musc Charleston Sc

Bivalent Covid 19 Vaccine Carecentral Urgent Care Massachusetts
Covid 19 Bivalent Vaccine Available At Uhc Uga Student Affairs